|
Non-locality--its supreme importance
Quantum theory has many strange quirks that are rightly labeled "out of this world." Of these, surely the most significant for us human beings is the Bell-Aspect proof of the reality of non-locality--that is a transcendent arena of reality outside of space-time--the existence of which constitutes the ultimate challenge to materialism.
Carried to a logical conclusion, non-locality implies the existence of a transcendent universal consciousness (i.e how does a mere photon "sense" the thickness of a barrier?)--and that consciousness is both within and beyond this material world. For material realists the alternative interpretations are:
To accept that there are faster-than-light signals in a transcendent realm in which hidden variables exist.
Either give up strong objectivity or else accept a role for the observer's consciousness.
Sweep the Bell-Aspect work under the carpet.
The Bell-Aspect results and their independent confirmation occurred more than 20 years ago. And although they shattered the foundations of materialism, they can provide a meaning for life, even open the pathway to God, and are by far the most significant achievements of quantum science for humanity up to this present day--nevertheless they remain ignored and almost unknown.
What most quantum physicists believe.
Back in 1911, Ernest Rutherford proposed a planetary model for atomic electrons which, he said, circulated around the atom's nucleus much as planets revolve about the sun.
However this model had a weakness in that it was inherently unstable and should eventually result in electrons crashing into the nucleus or being lost by its atom.
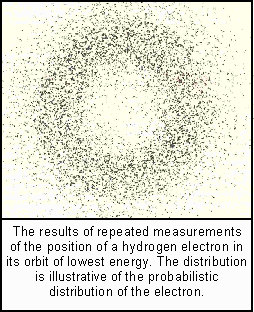
Suppose, said Norwegian physicist, Neils Bohr, that the orbits the electrons describe are discrete. Each such orbit, from the lowest energy level to the highest, has a fixed unalterable pathway--a stationary orbit, non-changing in its energy value. To change that orbit, energy must be absorbed or emitted in discrete quanta. But in doing so, it is by a quantum jump, without that electron ever being anywhere in between.
These electron orbits were also visualized as stationary waves, each of which, according to Max Born, was really a probability wave that tells us where we are likely to find an electron in any attempted observation. However, in order to do so for a single electron, the observer is forced to collapse the wave pattern. Thus single electrons can only ever be observed in particle form.
These concepts were slowly developed by physicists such as Heisenberg, Dirac, and Schrodinger--the wave equation for matter, known as the Schrodinger equation, emerging as the connection for the mathematics that replaced Newton's laws in the new physics.
The revolution in all this was that the change over from classical to quantum physics introduced uncertainty, for we can no longer think in terms of the absolute position and momentum of any object. Now, and presumably forevermore, we can only provide a probability estimate of such parameters, and these must be in accord with the Heisenberg uncertainty principle which states that the more accurately we know the position of an object, the less we can know about its momentum or velocity--and vice versa.
These are "uncertain" times in which the atom and its sub-atomic components belong to the quantum world--a world of components that exist in states of "being neither this nor that," and are dislodged from such states only when observed.
|
|